In another post, a discussion arose about whether it might be possible to trap cyclopropenylidene to form a small molecule with a large dipole moment. Doing so assumes that cyclopropenylidene has a sufficiently long lifetime to so react, before it does so with itself to e.g. dimerise. That dimerisation has an energy profile shown below, with a free energy of activation of 14.4 kcal/mol, so a facile reaction that will indeed compete with reaction with Ph-I+-CC–.
The schematic above shows some arrow pushing schemes for this reaction. In (a), one pair of electrons in the reacting carbene will have to be elevated into the π-system to form the π covalent bond, whilst the other pair of electrons will remain as σ and form a C-C σ-bond. One could do this in two stages. Firstly the double excitation of a carbene lone pair into the p-orbital and then the reaction between the two different electronic states of this species. In fact the IRC above shows no sign at all of a two-stage process; the reaction is entirely synchronous. An NBO analysis at the transition state for the reaction shows two equivalent carbene lone pairs each overlapping with one of the two empty p-orbitals on the original carbene. Click on the diagrams below to obtain rotatable 3D models of this overlap.
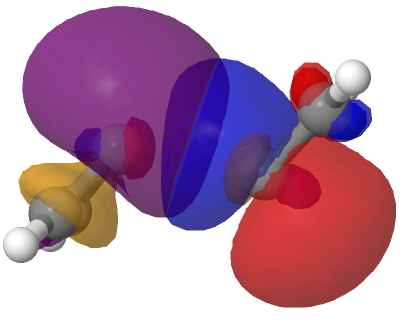
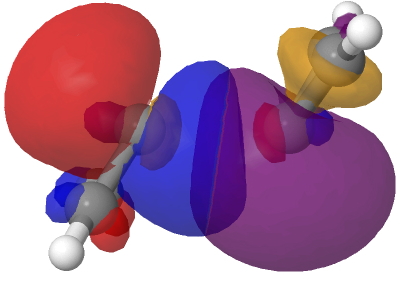
So perhaps representation (b) of this reaction might be as follows, in which each electron of the carbene lone pair does something different, one becoming π and one remaining σ. This reminds of how proton-coupled electron transfers are represented, in which the two electrons of an electron pair each do different things.[1]
Another way of thinking about it is not to form one σ- and one π-bond but to form two “banana bonds” as in (c), in which each of these bonds is equivalent. Banana bonds have rather gone out of vogue, largely because they do not illustrate why an alkene has different reactivity to an alkane. There will also be those who would dismiss these attempts on the grounds that “curly arrows” are merely a qualitative representation/book-keeping of the reaction and should not be used for implying quantum mechanical results. I happen to think otherwise, but the above does serve to illustrate that sometimes, the “curly arrows” for a reaction do need some thinking about!
References
- J.E.M.N. Klein, and G. Knizia, "cPCET versus HAT: A Direct Theoretical Method for Distinguishing X–H Bond‐Activation Mechanisms", Angewandte Chemie International Edition, vol. 57, pp. 11913-11917, 2018. https://doi.org/10.1002/anie.201805511
Would Et3C groups be bulky enough to block the dimerization?
Interesting question! There are two possible outcomes:
1. Dimerisation is easier
2. Dimerisation is more difficult.
The first is possible because the bulky groups may well attract each other due to dispersion effects, and hence make the dimerisation easier!
I will report back after my modelling of bulky groups has finished.
The answer to your question is now a separate post. More to come!
What happens if the carbene is coordinated to Li+? Making it by deprotonating (Me3C)2C3H+ seems reasonable. If done with a strong base like LiTMP there could be Li+ attached.