
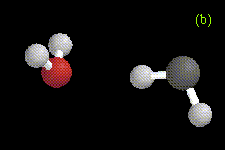
In our preliminary ab initio studies of complexes formed between methylene and water, methanol, or dimethylether, we have also found evidence for some interesting hydrogen-bonded species. Two different hydrogen-bonded complexes formed between methylene and water are shown in Figure 3, optimized at the MP2/6-31G* level. These configurations represent local minima on the potential energy surface. In the first conformation, one of the hydrogens on water is involved in a hydrogen bond to the carbene. In the second conformation, one of the hydrogens on methylene is involved in a hydrogen bond to water. Similar structures have also been found for hydrogen-bonded complexes between methanol and methylene at the RHF/3-21G level (Figure 4) and between dimethylether and methylene at the RHF/3-21G level (Figure 5). Not all the hydrogen-bonded structures have been located yet at the higher levels of theory. For the interaction between dimethylether and methylene, there is only one possible hydrogen-bonded complex due to lack of a hydrogen attached to the oxygen atom.

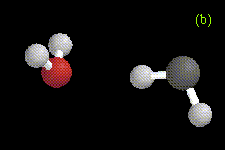
Figure 3. Optimized geometries at the MP2/6-31G* level for (a) Conformation 1, and (b) Conformation 2 of the hydrogen-bonded complexes formed between H2O and CH2. The hydrogen bond distance is 2.16 Å in Conformation 1, and 2.50 Å in Conformation 2.

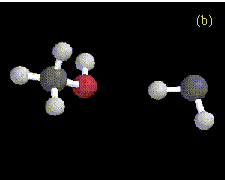
Figure 4. Optimized geometries at the HF/3-21G level for (a) Conformation 1, and (b) Conformation 2 of the hydrogen-bonded complexes formed between CH3OH and CH2. The hydrogen bond distance is 2.17 Å in Conformation 1, and 2.26 Å in Conformation 2.

Figure 5. Optimized geometries at the RHF/3-21G level for the hydrogen-bonded complex formed between CH3OCH3 and CH2. The hydrogen bond distance is 2.22 Å.
Binding energies for the hydrogen-bonded systems are presented in Table 3. Depending on the basis set and level of theory, the binding energies for these complexes range from 2 - 8 kcal/mol. These hydrogen-bonded complexes have binding energies that are much weaker than the ylide-like complexes discussed above.