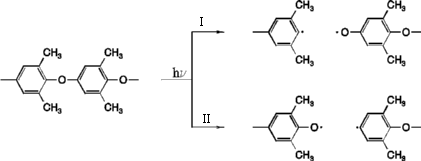
Scheme 1: Mechanism of ether bond cleavage

Scheme 1: Mechanism of ether bond cleavage
This intramolecular degradation mechanism was proposed by Rivaton and Morel [1]. The primary process is a cleavage of the ether bond leading to a phenyl and a phenoxy radical. This cleavage can occur on two different positions as can be seen in the scheme above. The results for both pathways are summarized in table 1 and figures 1 and 2. The energies of the formed products are summarized in table 2.
Optimized structures can be obtained here:
Cleavage I: Educt ---> Transition State ---> Product Cleavage II: Educt ---> Transition State ---> Product
Table 1: Calculated state energies for the
ether bond cleavage. Energies given in kJ/mol.
| Cleavage I | Cleavage II | ||
|---|---|---|---|
| Conformer | State | E(State) | E(State) |
| Educt | S0 | 0.0 | 0.0 |
| T1 | 339.1 | 339.1 | |
| S1 | 424.1 | 424.1 | |
| Transition state | S0 | 233.6 | 225.0 |
| T1 | 377.7 | 318.7 | |
| S1 | 519.3 | 505.1 | |
| Product | S0/T1 | 213.9 | 204.8 |
| S1 | 293.3 | 475.5 |
Table 2: Calculated UHF-AM1 ground state energies
of the products formed in the ether bond cleavage. Energies given in kJ/mol.
For structures select here.
Cleavage I: Phenyl- and
Phenoxyradical.
Cleavage II: Phenyl- and
Phenoxyradical.
| Cleavage I | Cleavage II | |
|---|---|---|
| Conformer | E(S0) | E(S0) |
| Phenylradical | 57.1 | 69.9 |
| Phenoxyradical | -218.8 | -240.7 |
 Fig. 1: Cleavage I
Fig. 1: Cleavage I
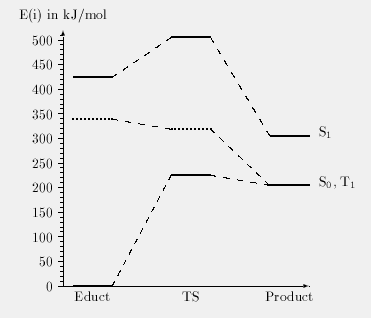 Fig. 2: Cleavage II
Fig. 2: Cleavage II
 Results Overview
Results Overview
