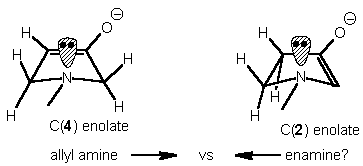
Experimental Conditions C(2) : C(4)
enolate(2) enolate(3)
LDA, TESCl, THF, -78 °C 1 : 1.3
LDA, TESCl, THF, -78 °C in situ 1 : >8
electrophile
LDA, TESCl, THF, -78 °C reverse 1 : >7
addition
nBuLi, HN(C6H10)2, -78 °C 1 : 1.7
LTMP, TESCl, THF, -78 °C 1 : 3.5
Garst et al(ref1): LDA, TESCl
THF, -78 °C 1 : 1
Regioisomeric composition calculated from the 1H NMR of the silyl
enol ethers.LTMP=lithium tetramethyl-piperidide,
