6 Boulevard du Marechal Juin, 14050 Caen, France
We were pleased to observe that addition of one equivalent of methyllithium to sulfines 2 at -78°C produced the stabilized anions 3, arising from selective thiophilic addition. Subsequent reaction of 3 with enones 4 occurred only in a conjugate fashion leading to adducts 5. Steric decompression around the sulfur substituted carbon occurred spontaneously via elimination of methanesulfenic acid to afford [[beta]]-oxoketenedithioacetals 6. Synthetic routes to these unconjugated moieties are scarce compared to the straightforward [[alpha]]-oxoketenedithioacetals.

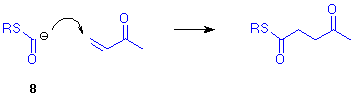
Further transformation can be achieved by an easy "hydrolysis" of ketenedithioacetal moiety to give the thiolester group. Thus we obtained 4-oxothiolesters 7 in good yields. These arise from the formal 1,4-addition of an alkylthiocarbonyl anion synthon to Michael acceptors. It should be noted that, in contrast to the now classical "Umpolung" reagents, the intermediate anions were prepared by addition and not by deprotonation, thus allowing further perspectives for use with multifunctional compounds.