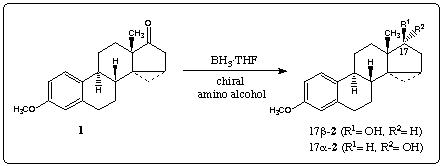

Reduction with diborane5 afforded an 85:15 mixture of 17β-2 und 17alpha-2 which was separated by chromatography. Treatment of 1 with borane and catalytic amounts of achiral 3, rac-46 and rac-57 gave the secondary alcohols in high yields (table 1, entry 1-3). These results show that the chirality of the substrate 1 forces the reaction to give alcohol 17β-2 in excess.
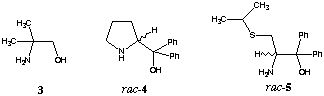
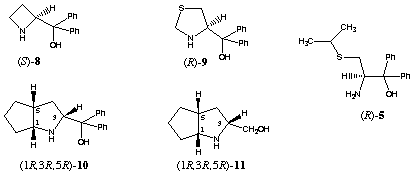
Borane reduction of 1 in the presence of homochiral amino alcohols (R)-48, (R)-69 and (R)-710 increased the stereoselectivity, providing 99% 17β-2 and 1% 17alpha-2 (entry 5). In this case we observed a large double asymmetric induction. This chiral double recognition could also be obtained with (R)-911, (1R,3R,5R)-1012 and(1R,3R,5R)-1113.
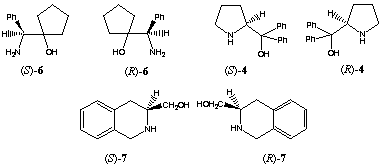
On the other hand 87% of the secondary alcohol 17alpha-2 was obtained by changing the configuration of the homochiral catalyst (entry 4). Thus, the amino alcohols (S)-414, (R)-515, (S)-616, (S)-717 and (S)-818 are able to superseed the intramolecular induction.
The stereochemistry of the new stereogenic center in 2 is affected mainly by the configuration of the homochiral amino alcohol. The catalyst can clearly control the stereoselectivity of the reaction to a large degree, although the chiral centers of the ketone 1 have a strong influence upon the diastereoselectivity in reductions with achiral or racemic amino alcohol.
Table 1. Diastereoselective reduction of 3-methoxy-14alpha-,15alpha-methylen-estra-1,3,5(10)- trien-17-on 1
entry catalyst catalyst temperature time yield [%] 17alpha-2:17beta-2a
concen-tration [&176;C] )
[mol%]
1 3 10 30 4 94 23:77
2 rac-4 10 30 4 94 37:63
3 rac-5 10 35 16 98 32:68
4 (S)-6 10 30 48 95 87:13
5 (R)-6 10 30 48 94 1:99
6 (S)-4 10 30 4 91 86:14
7 (R)-4 10 30 4 94 5:95
8 (S)-7 10 30 48 91 57:42
9 (R)-7 10 30 48 90 5:95
10 (S)-8 10 30 4 66 78:22
11 (R)-9 10 65 16 79 13:87
12 (R)-5 5 30 4 90 80:20
13 (1R,3R,5R)-10 10 30 48 91 8:92
14 (1R,3R,5R)-11 10 30 48 92 7:93
a
The ratio of the alkohols 17alpha-2:17beta-2 was determined
by HPLC analysis of the recovered residue.
In a typical procedure a mixture of the ketone 1 in dry THF was slowly added within 1 hour to a solution of the catalyst and borane-THF complex in dry THF at 30&176;C or 65&176;C. After stirring at the respective temperature the reaction mixture was hydrolyzed with 2 N HCl and extracted with MTBE. The combined organic layers were successively washed with 2N NaOH and NaCl solution, dried (MgSO4) and concentrated under reduced pressure. The obtained crude product was analysed by HPLC in order to avoid enrichment of one diastereomer by crystallization.
A summary is given below:

Acknowledgements : Thanks are due to Degussa AG, Hermann-Schlosser-Stiftung and Fonds der Chemischen Industrie for support.
2 (a) A. Hirao, S. Itsuno, S. Nakahama, N. Yamazaki, J. Chem. Soc. Chem. Commun. 1981, 315-317. (b) S. Itsuno, A. Hirao, S. Nakahama, Y. Yamazaki, J. Chem. Soc. Perkin I 1983, 1673-1676. (c) S. Itsuno, K. Ito, A. Hirao, S. Nakahama, J. Chem. Soc. Perkin I 1984, 2887. Reviews: (d) S. Wallbaum, J. Martens, Tetrahedron: Asymmetry 1992, 3, 1475-1504. (e) L. Deloux, M. Screbnik, Chem. Rev. 1993, 93, 763-784.
3 (a) I. Ojima, T. Suzuki, Tetrahedron Lett. 1980, 1239-1242. (b) I. Ojima, N. Yoda, Tetrahedron Lett. 1982, 3913-3916. (c) I. Ojima, N. Yoda, M. Yatabe, Tetrahedron Lett. 1982, 3917-3920. (d) H. Levine-Pinto, J. L. Morgat, P. Fromageot, D. Meyer, J. C. Poulin, H. B. Kagan, Tetrahedron 1982, 38, 119-123. (e) I. Ojima, T. Kogure, N. Yoda, T. Suzuki, M. Yatabe, T. Tanaka, J. Org Chem. 1982, 47, 1329-1334.
4 (a) K. Soai, C. Shimada, M. Takeuchi, M. Itabashi, J. Chem. Soc. Chem. Commun. 1994, 567-568. (b) K. Soai, K. Takashi, J. Chem Soc. Perkin Trans 1, 1994, 1257-1258. (c) M. Watanabe, K. Soai, J. Chem Soc. Perkin Trans 1, 1994, 3125-3128. (d) M. Okamoto, M. Tabe, T. Fujii, T. Tanaka, Tetrahedron: Asymmetry 1995, 6, 767-778.
5 R. Prousa, B. Schönbecker, D. Tresselt, K. Ponsold, J. prakt. Chem. 1986, 328, 55-70.
6 E. J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc. 1987, 109, 7925-7926.
7 T. Mehler, J. Martens, unpublished results.
8 E. J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc. 1987, 109, 7925-7926.
9 Ch. Dauelsberg, J. Martens, Synth. Commun. 1993, 23, 2091-2099.
10 K. Stingl, J. Martens, unpublished results.
11 W. Trentmann, J. Martens, unpublished results.
12 S. Wallbaum, J. Martens, Tetrahedron: Asymmetry 1991, 2, 1093-1096.
13 Ch. Döbler, U. Schmidt, H. W. Krause, H.-J. Kreuzfeld, M. Michalik, Tetrahedron: Asymmetry 1995, 6, 385-388.
14 E. J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc. 1987, 109, 5551-5553.
15 T. Mehler, J. Martens, Tetrahedron: Asymmetry 1993, 4, 2299-2302.
16 I. Reiners, J. Martens, unpublished results.
17 K. Stingl, J. Martens, S. Wallbaum, Tetrahedron: Asymmetry 1992, 3, 223-226.
18 W. Behnen, Ch. Dauelsberg, S. Wallbaum, J. Martens, Synth. Commun. 1992, 22, 2143-2153.