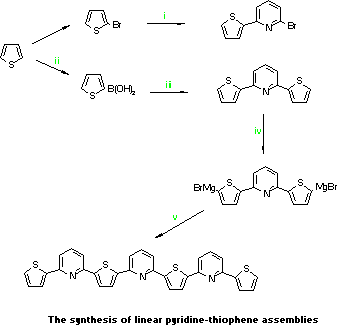
- Magnesium turnings (1.61g,
0.066gatoms) in dry THF (100mL) under nitrogen was treated with a
little of 2-bromothiophene (10.0g, 0.06mol) in THF (50mL). Warming
initiated the reaction and the remainder added over 45 in. After
3.5h and a further 15 min. reflux, the cooled solution (0-5 C) was
transferred dropwise by canula to a mixture of 2,6-dibromopyridine
13.08g, 0.055mol), PdCl2.dppe (0.5g) and dry THF (50mL) under
nitrogen at -5 C. After 10h at 30 C ether was added (100mL), the
solution washed with 4M HCl solution (sat., 150mL), then water. The
dried and evaporated solution yielded an oil which was distilled (b.p.
150 C/0.1mm) to give 2-bromo-6-(2-thienyl)pyridine as a colourless
liquid(8.5g, 59%).
- Thiophene (20.0g, 0.24mol) in dry THF (150mL)
at -40 C under nitrogen was treated with n-butyllithium (0.24mol),
stirred for 30 min. and then at -10 C for 30 min. This solution was
cooled to -50 C and trimethyl borate 88g, 0.85mol) added over 1h.
After a further 30 min. at -50 C the solution was warmed to ambient,
neutralised with 2M HCl ether extracted, and the ether layer extracted
3 times with 3M NaOH. The aqueous layer was acidfied at 0-10 C and
then extracted with ether (3x) and evaporated to give waxy white
solid (37.4g, 79%) used without further purification.
- A
mixture of 2,6-dibromopyridine (1.00g, 4.22 mmol),
tetrakis-(triphenyl- phosphine)palladium[0] (0.175g), toluene (30mL),
water 30mL), KF (1.0g) and thiophenboronic acid (2.5g) were refluxed
under nitrogen for 4h. The toluene layer was washed with water,
dried, evaporated and flash chromatographed (SiO2, PE/EtOAc 7:3) to
give ,6-bis-(2-thienyl)pyridine (TPT for short) (0.91g, 90%), m.p.
78.5-9.5 C.
- To Mg (0.30g, 0.012gatoms)in dry THF (20mL) under
nitrogen was added dropwise 1,2-dibromoethane (1.85g, 9.85mmol) in THF
(30mL) and the solution refluxed for 50 min. In a separate flask,
n-butyllithium (9.02mmol) was added to TPT (1.00g., 4.0mmol) in dry
THF (20mL) at -40 C under nitrogen and after a further 30 min. at -40
C warmed to 0 C for 30 min., cooled to -60 C and the above MgBr2
solution added. After 30 min. at -60 C the solution was warmed to
ambient to give the bis-Grignard reagent used directly as below.
-
To the above solution was added a solution of
2-bromo-6-(2-thienyl)pyridine (2.41g, 0.01mol) and PdCl2.dppb) in dry
THF 20mL) and the mixture refluxed for 4h. Removal of the THF and
addition of CHCl3 gave a solution that was washed with sat. NH4Cl then
water, dried and evaporated to give a yellow solid that was flash
chromatographed (SiO2, PE/CH2Cl2 6:4) to give TPTPTPT (1.24g, 57%) as
yellow crystals from ethyl acetate, m.p.117.5-8.5 C.
Back to main page

