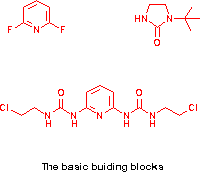
 2,6-Difluoropyridine unlike the 2,6-dichloro-analogue is quite reactive to
nucleophiles. The first F is easily replaced, often at ambient
temperatures, the second is thereby deactivated and requires more vigorous
conditions (q.v.).
Imidazolidinone is a cheap and useful building block. With t-butanol and
conc. H2SO4 it is easily converted into the beautifully crystalline mono
t-butyl derivative (62% on a 60g scale) , m.p. 138.5-139.5 C (ref 1) m.p.
135-136C. The imidazolidinone (60.0g finely powdered) is added slowly to
conc. H2SO4 (75mL) at 0-15 C and then t-butanol (125mL) added dropwise at
20-25 C with stirring. After a further 30min. the mixture is poured onto
ice (~1Kg) neutralised below 25 C, cooled to 15 C and extracted with CHCl3.
The crude product (45g) is recrystallised from ethyl acetate/petrol.
2,6-Diaminopyridine is also cheap and easily transformed into the
2,6-chloroethylurea-derivative by the action of chloroethyl isocyanate as
follows: To 2,6-diaminopyridine (7.80g) in THF (50mL) was added dropwise at
0-5 C chloroethylisocyanate (17.10g) in THF (50g). The mixture was then
stirred at ambient overnight. Removal of the solvent gave
2,6-bis-(N-[2-chloroethyl)-N'-ureido)pyridine as white plates from ethanol
(17.50g, 78%), m.p. 169-170 C [ref 2 m.p. 171-4 C].
2,6-Difluoropyridine unlike the 2,6-dichloro-analogue is quite reactive to
nucleophiles. The first F is easily replaced, often at ambient
temperatures, the second is thereby deactivated and requires more vigorous
conditions (q.v.).
Imidazolidinone is a cheap and useful building block. With t-butanol and
conc. H2SO4 it is easily converted into the beautifully crystalline mono
t-butyl derivative (62% on a 60g scale) , m.p. 138.5-139.5 C (ref 1) m.p.
135-136C. The imidazolidinone (60.0g finely powdered) is added slowly to
conc. H2SO4 (75mL) at 0-15 C and then t-butanol (125mL) added dropwise at
20-25 C with stirring. After a further 30min. the mixture is poured onto
ice (~1Kg) neutralised below 25 C, cooled to 15 C and extracted with CHCl3.
The crude product (45g) is recrystallised from ethyl acetate/petrol.
2,6-Diaminopyridine is also cheap and easily transformed into the
2,6-chloroethylurea-derivative by the action of chloroethyl isocyanate as
follows: To 2,6-diaminopyridine (7.80g) in THF (50mL) was added dropwise at
0-5 C chloroethylisocyanate (17.10g) in THF (50g). The mixture was then
stirred at ambient overnight. Removal of the solvent gave
2,6-bis-(N-[2-chloroethyl)-N'-ureido)pyridine as white plates from ethanol
(17.50g, 78%), m.p. 169-170 C [ref 2 m.p. 171-4 C].
References:
1. H. Quast and U. Nahr, Chem. Ber., 1984, 117, 2761.
2. T. P. Johnson and G. S. McCaleb, J. Med. Chem., 1966, 9, 892.
Back to main page

