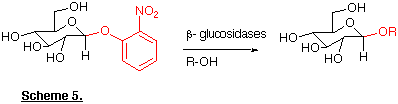
Hydrolysis of Racemic Esters
Kinetic Resolutions

Kinetic resolutions of selected esters have been used in the preparation of a number of important compounds in these laboratories including a synthon for the hypocholestemic delta-lactones (Olivo et al., 1993), anti-AIDS agents (McCague et al., 1994) and the fascinating anti-fungal agent called brefeldin - A (Carnell et al., 1994).
Asymmetrization of Meso-Esters
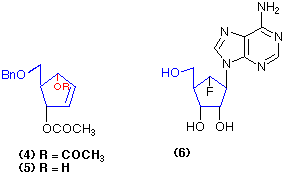
Similarly the dimethyl cyclopentane-dicarboxylate (7) is hydrolysed with exquisite selectivity using pig liver esterase to give the mono-ester (8) in a highly pure form (96% yield; 98% e.e.) (Cotterill et al., 1991a). Enzyme-catalysed reactions such as (4) => (5) and (7) => (8) give the possibility of forming optically pure products in quantitative yield from the chosen substrates, a process that is very difficult to emulate using conventional chemical catalysts.

Esterification of Racemic Acids
Roberts, 1989). For example, the racemic alcohol (9) in hexane is
converted into the optically active ester (11) (90% e.e.) and recovered
optically enriched bicyclo[3.2.0]hept-2-en-6endo-ol using cyclohexane
carboxylic acid and the catalyst Lipozyme® (Mucor miehei lipase
attached to an inert solid support) (Cotterill et al., 1988a).
Inter-Esterification

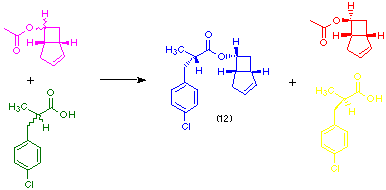
The inter-esterification process has recently been extended to provide an example of double enantioselection by using the ester (±)-10 and racemic 2-(para-chlorophenoxy)propanoic acid. One of the four possible diastereoisomeric esters, compound (12), is formed almost exclusively (Fowler et al., 1991). Two stereoselective enzyme-catalysed processes are in operation, namely the preferential hydrolysis of the 6(R)-acetate (10), and selective acylation of the product alcohol by 2(-)-(para-chlorophenoxy)-propanoic acid.
Dynamic Resolution

Polyesters
The use of lipases for the preparation of polyesters has been studied at
Exeter (Binns et al., 1993) and the condensation of adipic acid and
butane-1,4-diol has been developed, by an industrial partner, to form part of a
large scale process for the production of polyurethanes.
Amide Hydrolysis
The employment of acylases (such as hog kidney acylase) for the cleavage of
amide bonds under mild conditions is well known. This type of hydrolysis is
commercially important in the preparation of 6-amino-penicillanic acid and in
the synthesis of some optically pure amino-acids.
More recently it has been found that a microbial acylase can effect the enantiospecific hydrolysis of the lactam (16) to give the amino-acid (17) and recovered starting material. A second microorganism can effect the enantio-complementary hydrolysis to give the mirror image of compound (17) and recovered lactam (Taylor et al., 1990). These optically active amino-acids and lactams can be used to prepare the important anti-AIDS agent carbovir (18) in homochiral form. The synthesis of the GABA-inhibitor (19) from both enantiomers of the lactam (16) in an enantio-convergent strategy exemplifies another important application of this useful enzyme-catalysed kinetic resolution (Evans et al., 1991).

Phosphate Esters
The synthesis of phosphate esters can be accomplished by kinase enzymes
using adenosine triphosphate (ATP) as the phosphate donor. The technique will
work with unnatural substrates: for example the racemic nucleoside analogue
(20) was converted into the racemic nucleotide (21) using thymidine kinase and
ATP. Enantioselective hydrolysis of the (±)-phosphate (21) can be achieved
using 5'-nucleotidase from snake venom to give the dextrorotatory carbocyclic
nucleoside (+)-(20) which exhibited very powerful anti-Herpes activity
(Borthwick et al., 1988 and 1990).
Nitrile Hydrolysis
The hydrolysis of nitrile groups using hydratase enzymes is of great interest
to many synthetic organic chemists, principally because the hydrolysis takes
place under very mild conditions (for a recent review see Crosby et al.,
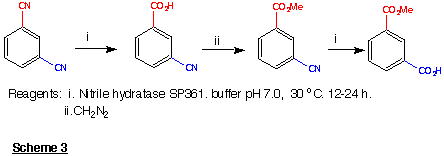
1994) The sequence outlined in Scheme 3 (Cohen et al., 1990) illustrates
the selectivity of processes of this type (see also Kakeya et al.,
1991).
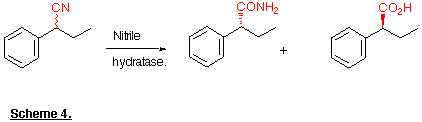
Recently it has been shown that this reaction can be carried out in an enantioselective fashion (Kakeya et al., 1991; Cohen et al., 1992) in which the resolution occurs during the amide to acid conversion. For example racemic 2-phenylbutyronitrile yields (R)-2-phenylbutyramide and (S)-2-phenylbutyric acid, both with > 98% e.e. (Scheme 4). The asymmetric hydrolysis of 3-substituted glutaronitrile derivatives has also been achieved (Beard et al., 1993; Kerridge et al., 1994).
Epoxide Hydrolases
The substrate selectivity of epoxide hydrolase enzymes is being explored. To
date most of the biotransformation systems have been derived from animal
sources (e.g. rabbit or rat liver microsomes) and so the methodology is
not at all useful to the non-specialist wishing to prepare optically active
vic-diols on a reasonable scale.
Enzyme Models for Use by Non-Specialists.
The use of hydrolase enzymes by the non-specialist is aided by the
availability of models of the active site of enzymes such as pig liver
esterase, porcine pancreatic lipase, Pseudomonas fluorescens lipase and
Candida cylindracea lipase (Toone et al., 1989;
Oberhauser et al., 1989;
Santianello et al., 1988).
Glycosidases
Finally, glycosidases are a diverse group of enzymes whose natural function is
to catalyse the hydrolytic cleavage of glycosides. Recently we have exploited
the inherent selectivity of one particular glycosidase, ß-glucuronidase,
in the synthesis of the potent analgesic morphine-6-glucuronide (23). Treatment
of morphine-3,6-diglucuronide (22) with ß-glucuronidase from
Patella vulgata results in regioselective hydrolysis of the more reactive
3-glucuronide (Brown et al., 1995).
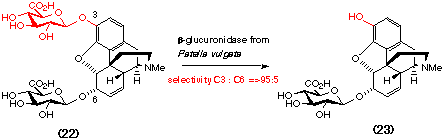
In addition it has long been known that these enzymes can also catalyse 'transglycosylation' reactions resulting in the formation of novel glycosides (Scheme 5). By using certain amino acid derivatives as the nucleophilic alcohol component it is possible to prepare carbohydrate-amino acid linkages (e.g. 24) that are important constituents of many glycoproteins (Turner and Webberley, 1991).