| [Related articles/posters: 110 032 023 ] |

| Electrophile | E | Product | Yield | Starting material 1 |
| MeCHO | MeCH(OH) | 3 | 55 % | 9 % |
| PhCHO | PhCH(OH) | 4 | 66 % | 12 % |
| Ph2CO | Ph2C(OH) | 5 | 78 % | - |
| PhSSPh | SPh | 6 | 82 % | - |
| HCONMe2 | CHO | 7 | 26 % | 10 % |
| HCO2Et | CHO | 7 | 19 % | 9 % |
| N-Formylpiperidine | CHO | 7 | 30 % | 10 % |
| ClSiMe3 | SiMe3 | 8 | 45 % | - |
| MeI | Me | 9 | 56 % | 14 % |
| CO2 | COOH | 10 | 24 % | - |
| I2 | I | 11 | 73 % | - |
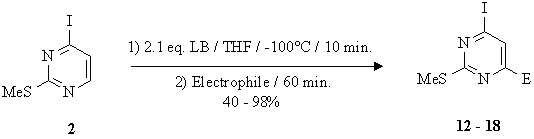 Electrophile Electrophile |
E | Compound | yield |
| CH3CHO | CH3CH(OH) | 12 | 96% |
| PhCHO | PhCH(OH) | 13 | 63% |
| Ph2CO | Ph2C(OH) | 14 | 72% |
| MeI | Me | 15a | 50% |
| Et | 15b | 24% | |
| ClSiMe3 | SiMe3 | 16 | 75% |
| HCO2Et | CHO | 17 | 40% |
| I2 | I | 18 | 57% |
| " | I | 18 | 98%* |
*Reaction was performed with 120 minutes for reaction time of iodine with lithio derivative
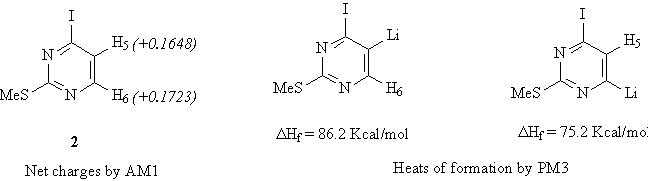 The heats of formation of lithiated intermediates, determined by semiempirical method Li/PM3 indicate that the C6 lithio derivative is more stable than those at C5, moreover the steric hindrance of the metalling agent could be also in favour of the regioselectivity at C6. These considerations are in agreemant with the unexpected regioselectivity at C6.
The heats of formation of lithiated intermediates, determined by semiempirical method Li/PM3 indicate that the C6 lithio derivative is more stable than those at C5, moreover the steric hindrance of the metalling agent could be also in favour of the regioselectivity at C6. These considerations are in agreemant with the unexpected regioselectivity at C6.