| Substituent | Position 2 | Position 3 |
| Methyl | 7.8 | -6.2 |
| Trimethylsilyl | 15.6 | 10.4 |
| Methylthio | 14.8 | -0.6 |
| Bromo | 12.7 | 2.4 |
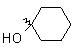 |
-2.8 | -7.6 |
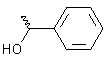 |
-1.2 | -6.2 |
| Aldehyde | -0.1 | 6.7 |
| Substituent | Position 2 | Position 3 |
| Methyl | 7.8 | -6.2 |
| Trimethylsilyl | 15.6 | 10.4 |
| Methylthio | 14.8 | -0.6 |
| Bromo | 12.7 | 2.4 |
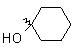 |
-2.8 | -7.6 |
 |
-1.2 | -6.2 |
| Aldehyde | -0.1 | 6.7 |
It is well known that 17O NMR is very sensitive to steric interactions [10].
This effect caused some deviations when using the above increments to calculate 17O-shifts of furans with ortho
substitution patterns. As these deviations are nearly constant for all compounds with identical
neighbouring groups, a steric correction can be introduced for each pair of substituents. Some examples are given below:
| Subst. in posn. 2 | Subst. in posn. 3 | Steric correction |
| Bromo | Bromo | -3.0 |
| Methyl | Bromo | -1.5 |
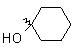 |
Bromo | + 4.0 |
| Bromo | Trimethylsilyl | + 2.8 |
| Bromo | 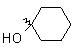 |
+ 6.8 |
By this method the furan oxygen shift can be calculated with a maximum deviation of ca. 2 ppm, provided the steric
correction - if necessary - is known. This can be illustrated by the following example:
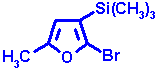
| Measured shift | 271.3 ppm | |
| Furan parent | 237.5 | |
| 2-Bromo | + 12.7 | |
| 2-Methyl | + 7.8 | |
| 3-Trimethylsilyl | + 10.4 | |
| Steric correction | +2.5 | |
| Calculated value | 270.9 ppm |