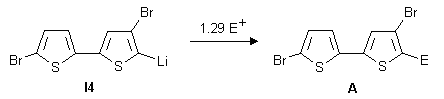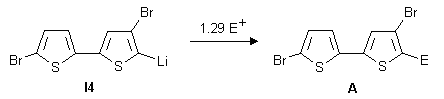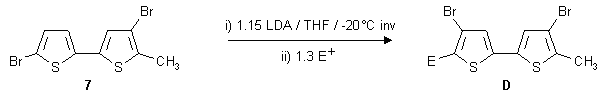Synthesis of migration products
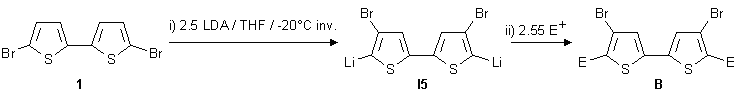
For preparative purposes, to a well-stirred suspension of 1.00 g 5,5'-dibromo-2,2'-bithiophene
1 in dry THF, 1.25 equiv. (2.50 equiv. resp.) LDA were added over 15 min via a
double ended needle at -20 °C.
Quenching the final lithio intermediates I4 and I5 respectively with various electrophiles
(CH3OH, TMSCl, TBDMSCl, cyclohexanone, DMF, CH3I) leads to compounds
2 to 15.
Scale up to multiple gram amounts became possible by utilizing a
specially designed reactor, which was used to add previously synthesized LDA at a constant rate.
Time of addition was increased proportionally to the upscaling-factor.
Mono-migration products 2-8
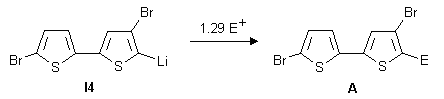
Mono-migration products
| Product |
Electrophile |
E |
Yield (%) |
| 2 |
MeOH |
H- |
68 |
| 3 |
TMSCl |
TMS- |
86-89 |
| 4 |
TBDMSCl |
TBDMS- |
81-91 |
| 5 |
Cyclohexanone |
1-Cyclohexanol- |
65 |
| 6 |
DMF |
-CHO |
63 |
| 7 |
Methyliodide |
-CH3 |
60-76 |
| 8 |
Dimethyldisulfide |
-SCH3 |
51 |
Double-migration products 9-15
Double-migration products
| Product |
Electrophile |
E |
Yield (%) |
| 9 |
MeOH |
H- |
60-74 |
| 10 |
TMSCl |
TMS- |
69-79 |
| 11 |
TBDMSCl |
TBDMS- |
71 |
| 12 |
Cyclohexanone |
1-Cyclohexanol- |
53 |
| 13 |
DMF |
-CHO |
67 |
| 14 |
Methyliodide |
-CH3 |
83 |
| 15 |
Dimethyldisulfide |
-SCH3 |
61 |
Mixed-migration products 16-20
Apart from mono-electrophilic substituted target compounds it also became possible to approach
"mixed"-migration products via two consecutive mono-migrations. One example starting from
the mono-rearranged methyl-compound 7 is shown below.
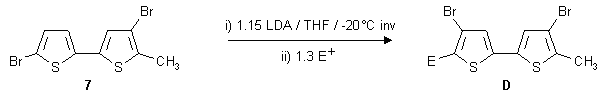
Mixed-migration products
| Product |
Electrophile |
E |
Yield (%) |
| 16 |
MeOH |
H- |
81 |
| 17 |
TMSCl |
TMS- |
64 |
| 18 isolated as 21 |
Cyclohexanone |
1-Cyclohexanol- as cyclohex-1-enyl- |
54 |
| 19 |
DMF |
-CHO |
70 |
| 20 |
Dimethyldisulfide |
-SCH3 |
70 |