Alkylation of pyrazolones via the Mitsunobu reaction
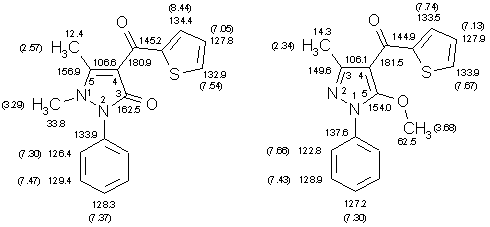
Spectroscopic investigations
Structures of compounds 5 and 6 were confirmed by spectroscopic methods (IR, MS, NMR).
Detailed 1H and 13C NMR spectroscopic studies were undertaken with N- and O-methyl
derivatives of pyrazolones 4. Unambiguous assignments for all carbon and proton resonances could be
achieved on basis of NOE difference experiments, fully 1H-coupled 13C NMR spectra (gated decoupling),
HMQC spectra [5], and long-range INEPT experiments with selective DANTE
excitation [6]. As a typical example a pair of corresponding N- and
O-methyl derivatives are shown below. 1H Chemical shifts are in
parentheses, numbers without parentheses refer to 13C chemical shifts. Worthy of note seem the facts that the
signals of the phenyl protons H-2/6 are remarkably less shielded in the O-methyl derivative (obviously
due to the influence of the lone-pair of the sp2-hybridized nitrogen atom N-2) as well as the large difference in the 1H chemical shifts of thiophene H-3 (this is a hint that in the preferred conformation of the N-methyl compound the thiophene H-3 comes close to the pyrazolone C=O moiety).