The best known example of the gauche effect is 1,2-difluoroethane, which exhibits a relatively small preference of ~0.5 kcal/mol for this conformer over the anti orientation, which is also a minimum. But FSSF, which I discussed in the previous post, beats this hands down! It also, by the way, must surely be the smallest molecule (only four atoms) which could be theoretically resolved into two enantiomers (possibly at say 273K?).‡
From this optimised scan[1] of the F-S-S-F torsion angle, you can see two striking differences
- Only the gauche form is stable. The anti form is in fact a transition state[2] for enantiomerisation of the two chiral C2-disymmetric gauche forms.
- The difference in free energy between the gauche form and the anti is 25.3 kcal/mol, compared with which the 0.5 kcal/mol for 1,2-difluoroethane looks puny indeed.
- The effect arises, as with difluoroethane, from overlap of the filled p-lone pair on one sulfur, with the accepting S-F σ* orbital.
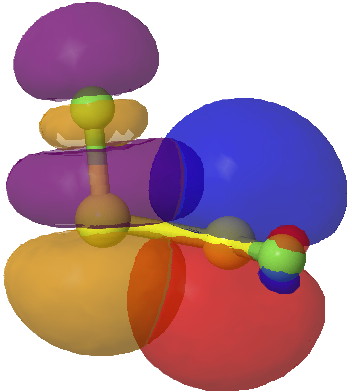
Click for 3D.
- This orbital overlap results in an NBO E(2) interaction energy of 39 kcal/mol. This compares with 5.6 kcal/mol for the equivalent C-H/C-F* term for difluoroethane, and it is of course larger because an S lone pair is a far better donor than a C-H bond. It is also far greater than the anomeric effect, which normally weighs in at about 16 kcal/mol.
- There is of course an alternative (and perhaps more unusual) transition state[3] for interconverting the two enantiomers of F-S-S-F which I described previously for F-S-S-Cl as involving a [1,2] migration of F. It however is 23.5 kcal/mol higher in energy than this pure bond rotation. Whereas the [1,2] F migration contracts the S-S bond at the transition state, the bond rotation lengthens it (from 1.922 to 2.142Å). This arises because the partial double bond character for the S-S bond is destroyed by rotation. The challenge then is whether one can find a 4-atom system where enantiomerisation proceeds by a lower energy continuously-chiral [1,2] migratory pathway rather than just by a simple bond rotation.
- An alternative visualisation of the electronic effects resulting in an extreme gauche effect can be seen from the ELF analysis[4] of the lone pair basins;
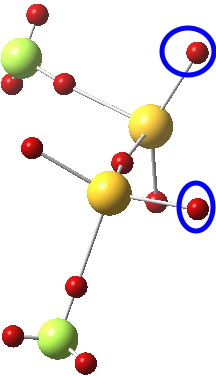
The two basins ringed in blue (2.25e) are each aligned at an angle of 167° to the axis of the antiperiplanar S-F bond. The knock-on effect of this is that the two lone pairs on each sulfur themselves subtend an unusual angle of 145° at the common sulfur, almost diaxial in fact.
I again marvel at how just four atoms and just two elements, can teach us so much chemistry!
References
- H.S. Rzepa, "Gaussian Job Archive for F2S2", 2013. https://doi.org/10.6084/m9.figshare.804328
- H.S. Rzepa, "Gaussian Job Archive for F2S2", 2013. https://doi.org/10.6084/m9.figshare.805048
- H.S. Rzepa, "Gaussian Job Archive for F2S2", 2013. https://doi.org/10.6084/m9.figshare.802815
- H.S. Rzepa, "Gaussian Job Archive for F2S2", 2013. https://doi.org/10.6084/m9.figshare.804332
Tags: ELF, Transition state

I have computed the Wiberg bond order indices for F-S-S-F. The S-S bond order is 1.47, and the S-F 0.70. This matches the relatively high barrier for S-S rotations.
I mentioned that F-S-S-F has disymmetric chirality. For the (M) enantiomer, one can compute the following chiroptical properties: the specific rotation is +247°589, +820°365.
Note the vibrational circular dichroism has a very strong Δε, the symmetric and antisymmetric F-S stretches being respectively negative and positive.